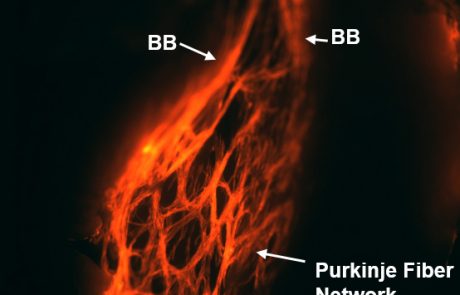
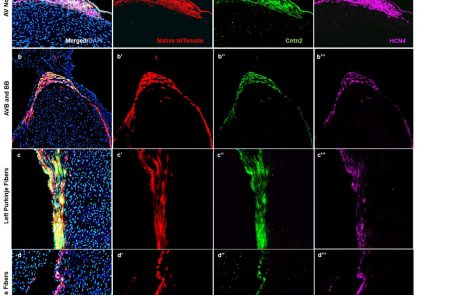
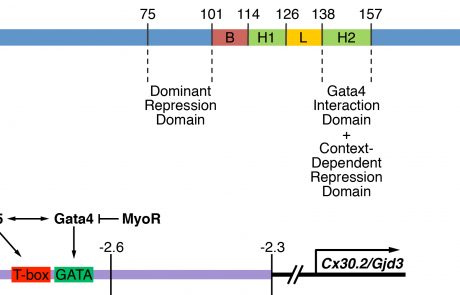
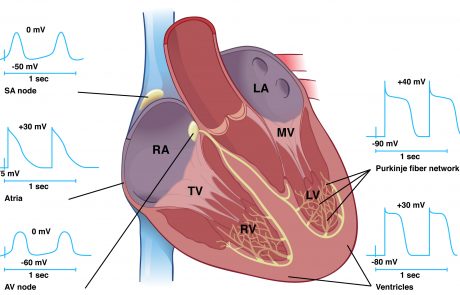
Development and function of the cardiac conduction system
We are interested in understanding the fundamental mechanisms responsible for generating normal cardiac rhythm. We believe this is an important step towards establishing the underlying pathophysiology for dysrhythmia and developing new therapeutics. Therefore, we use mouse genetics to study cardiac conduction system morphogenesis and function. Towards this aim, we have developed a series of unique mouse molecular tools to perturb gene expression within the conduction system, and we are developing unbiased approaches to map tissue-specific regulatory elements genomewide.
Click to view larger
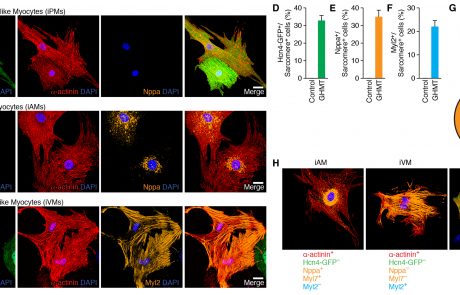
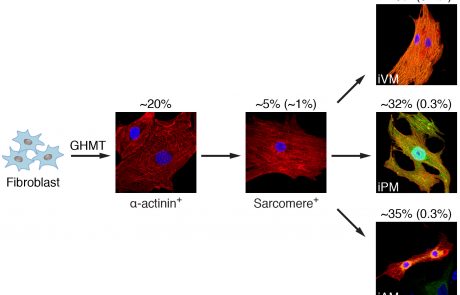
Generation of conduction cells by direct lineage reprogramming
Forced lineage conversions have literally revolutionized biology and paved the way for on-demand therapeutics. We are currently leveraging our growing insights into conduction system development combined with the broad expertise of our collaborators to create a biological pacemaker via directed lineage reprogramming.
Click to view larger
Cardiac conduction system regeneration
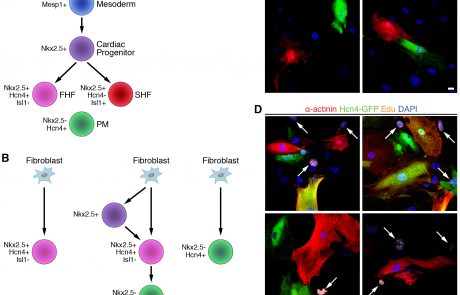
The capacity for tissue regeneration is directly related to its underlying proliferative capacity. Although the adult heart does not proliferate substantially, recent studies have identified a neonatal window during which cardiac regeneration is possible. In contrast to atrial and ventricular cardiomyocytes, however, the vast majority of conduction cells exit the cell cycle during embryonic development. Nevertheless, we are hopeful that conduction cells retain some degree of regenerative potential, so we are actively exploring this question using novel mouse models for inducible conduction cell ablation that we have developed.
Improving therapeutic delivery systems
The biological sciences community has now amassed an impressive toolkit for potentially attacking a broad variety of acquired and genetic diseases, yet a major stumbling block is “scar-less” therapeutic delivery. Although viral delivery is highly efficacious, we envision that effective, non-viral delivery methods are necessary for human translation. Therefore, we are developing novel strategies for delivery cells and macromolecules for therapeutic benefit.